Process 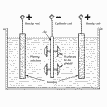
Zinc & Nickel-plating Zinc-plating is used to improve
corrosion resistance of steel components. There are 4
different methods of carrying out the process: using
high cyanogen, low cyanogen, basic or low acid
electrolytes.
Ni-plating is used for decoration and corrosion
protection of steel, Cu, Al and Mg; for leveling and
smoothing out surfaces; for black coatings; for
renowing worn machines components. Most
Ni-plating is applied prior to Cr-plating.
Two different electrolytes are used. The first, Watt's
solution, is based on Ni-sulphate, Ni-chloride and
boric acid. The second, Ni-sulphamate, is based on a
salt-Ni-sulphamate.
Danish NameCategoryMaterialsTypical
productsWater tap (Ni)
Cover
ReferencesDegussa AG - Geschäftsgebiet Galvanotechnik, (Germany)
Price notes| DKK/part | 5 parts | 100 | 5000 |
| Small (1cm2) | 85/85 | 11/11 | 0.056/0.077 |
| Medium (1dm2) | 115/117 | 20/22 | 0.40/0.78 |
| Large (100dm2) | 200/270 | 85/155 | 26/64 |
Price datePrice notesEnvironmental
notesAdditional
info