Process 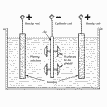
| Bronze/brass & other alloys plating Alloys-platings are
used when properties which cannot be spontanousely
aquired by the pure metal coatings are required. Two
or more metals fall simultaneously to the cathode.
Control of the bath chemistry and alloys' composition
is very expensive.
The most common alloys are: brass (Zn-Cu 70%) for
decoration and for improving the adhesion between
steel and rubber; Cu-Sn (90% Cu, bronze), against
adhesive wear, high costly; Cu (40-45%), corrosion
resistance;
Pb-Sn, Sn (6%), for corrosion protection; Sn (60%),
soldering metal; Ni-Fe(40%) to replace Ni when too
costly; Ni-Sn (65%), for corrosion resistance even in
very highly acid environments.
|
Danish Name | Elektrolytisk udfældning af legeringer |
Category | Surface coatings, Electrodeposited coatings
|
Materials | Coating materials:, Cu-Sn (bronze), Cu-Zn (brass), Pb-Sn, Ni-Fe,
Ni-Sn |
Typical
products | Water tap (bronze)
|
References | Arbeitsgemeinschaft der Deutschen Galvanotechnik, (Germany)
|
Price notes |
| DKK/part | 5 parts | 100 | 5000 |
| Small (1cm2) | 85/85 | 11/11 | 0.056/0.072 |
| Medium (1dm2) | 115/116 | 20/21.5 | 0.44/0.56 |
| Large (100dm2) | 210/245 | 95/127 | 30/43 |
|
Price date | 01-11-96
|
Price notes | The first price on the cell refers to bronze-plating, the second one to
brass-plating. Over 5000 parts the price doesn't vary so much. The price
is calculated for typical average thichnesses: 15 micron (bronze); 3
micron (brass).
|
Environmental
notes | In many cases it is used a cyanogen electrolyte, therefore very
poisonous. |
Additional
info | All alloys-plating processes are very costly because they require very
expensive equipment and professional staff. Labour is the most important
factor for pricing. |
